Specifying the bleeding disorder service
The blood disorders CRG has published a service specification for haemophilia services. This defines what is required for core service standards (that all providers should adhere to) and developmental service standards (that ‘may require further changes in practice over time to provide excellence in the field’).
The service specification covers haemophilia and other bleeding disorders, specifically:
- Hereditary factor VIII deficiency
- Hereditary factor IX deficiency
- Von Willebrand’s disease
- Hereditary factor XI deficiency
- Hereditary deficiency of other clotting factors
- Haemorrhagic disorder due to circulating anticoagulants
- Coagulation defect, unspecified
- Qualitative platelet defects
- Haemorrhagic condition, unspecified
The service specification is a comprehensive list of criteria that a service must meet if it is to receive an NHS contract. These cover the aims and objectives of the service, the care pathway, childcare, pregnancy, the population covered, acceptance and inclusion criteria, relationships with other services (such as rheumatology, women’s services, dentistry, and HIV and hepatology services), relevant national standards (such as NICE or guidance from a Royal College), and service outcomes. It states how treatments should be provided (but not which treatments) and the range of services that are needed to provide a service of the required quality. For example, a haemophilia service should be provided by experienced staff, nurses and physiotherapists with specialist training, appropriate lab support, genetic counselling, and access to a range of complementary services such as dentistry and social care.
The performance of providers is monitored against the outcomes in the service specification according to the criteria defined in a Specialised Services Quality Dashboard (SSQD). NHS Trusts and other providers monitor patient care and submit data on these indicators to NHS England for evaluation by specialised service commissioners.
The current SSQD for haemophilia includes four domains that derive from the NHS’s general objectives for patient care, an explanation of their value (Table 1), and instructions on how they should be measured and monitored.
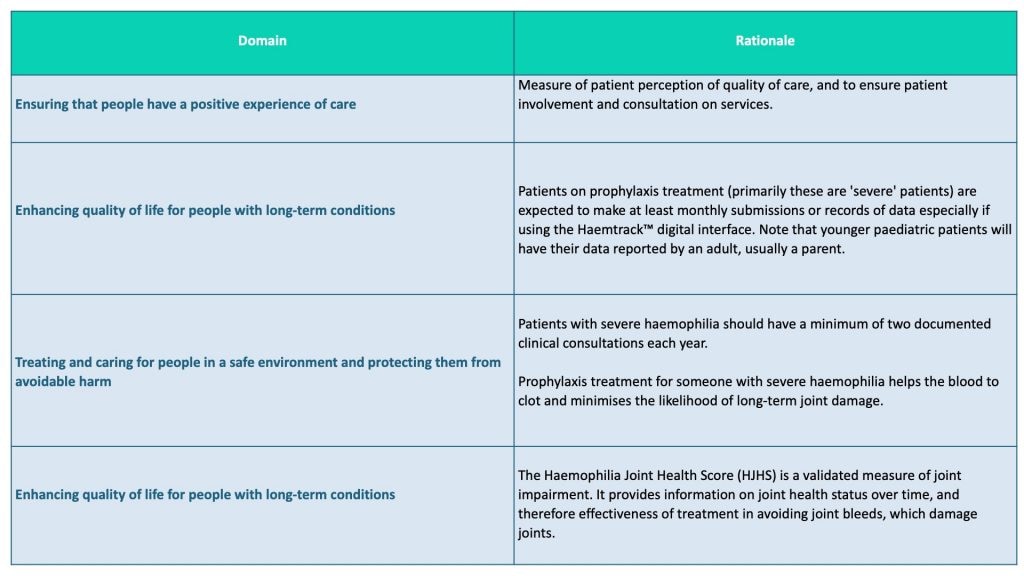
Table 1. Extract from NHS England Specialised Services Quality Dashboard for haemophilia, 2018-19
(https://www.england.nhs.uk/wp-content/uploads/2018/03/haemophilia-metric-definitions-2018-19-v1.pdf)
CRG prescribing policies
CRGs identify specialised treatments that they think should be considered for NHS funding. Their proposals are evaluated by the Clinical Priorities Advisory Group (CPAG) ‘according to their clinical effectiveness, benefit for patients and value for money’. In turn, CPAG makes recommendations to the Specialised Commissioning Oversight Group, which looks at the resource implications. The final decision as to whether or not to fund a specialised treatment is made by the Specialised Commissioning Committee, which is a subgroup of the NHS England Board.
The CRG for specialised blood disorders has also developed several policies that should be included in the relevant commissioning policy. These define the requirements for a service for a particular group of patients, ensuring consistency with NICE guidance and equal access to treatment across the country. All five of the policies currently listed online are about the treatment of haemophilia:
- Human coagulation factor X for hereditary factor X deficiency (all ages) (effective until 31 March 2019) and Human coagulation factor X for hereditary factor X deficiency (all ages) (effective from 1 April 2019)
- Emicizumab as prophylaxis in people with congenital haemophilia A with factor VIII inhibitors
- The use of Rituximab as a second line agent for the eradication of inhibitors in patients with Acquired Haemophilia
- Immune Tolerance Induction (ITI) for haemophilia A (all ages)
Taking the 2018 policy for emicizumab as an example, this was developed in collaboration with NICE and specifically covers prophylaxis in people of any age with congenital haemophilia A who have inhibitors.
The emicizumab policy does not apply to individuals with acquired haemophilia or to those who do not have inhibitors. It includes a plain English summary, which might be helpful when explaining treatment options to a patient. The document then provides a brief summary of the treatment of inhibitors with bypassing agents, a glossary, a needs assessment and a summary of the evidence of effectiveness and safety (with references).
The information of direct relevance to commissioning accounts for only four of the document’s 25 pages. This includes the criteria for starting and stopping treatment with emicizumab, where it fits in the treatment pathway, governance arrangements, a brief statement of about funding, requirements for audit and a list of relevant policies on which the policy is based.
Limitations of specialised commissioning and alternatives
The five policies cover only a small proportion of clinical activity in a haemophilia service, most of which is determined by other standards and guidelines from NICE and various professional bodies. The policies are developed to manage developments in clinical practice, restricting reimbursement for prescribing to indications that can be shown to represent value for money for the NHS. They are normally limited to a product’s licensed indications. From the perspective of the NHS, this approach offers a balance of controlling spending and meeting the needs of patients. The NHS has a horizon-scanning process by which it monitors the development of new medicines; by the time a new product is launched, the NHS should know how many patients will be treated and at what cost. From the patient’s perspective, the process may be perceived as delaying access to treatment if a policy is not in place by the time a new medicine is licensed.
Funding to use a medicine for an unlicensed indication is available via a different pathway. An example of when this would apply is currently (January 2019) the use of emicizumab for haemophilia A in a person who does not have inhibitors. Treatment may be available via participation in a clinical trial, or to an individual who was in a trial that has ended and is now receiving treatment on compassionate grounds (because it would be unreasonable to stop it and compel the person to have what may be inferior treatment). The alternative mechanism for funding through the NHS is to show that exceptional use is justified in each case separately by making an individual funding request (IFR).
NHS England defines treatments for exceptional use as ‘treatments which are not currently routinely commissioned or subject to a mandated guidance from NICE’. To access funding, a clinician must successfully argue for ‘approval when others from the same patient group are not being funded for the same treatment’. This procedure is strictly for individuals ‒ if ‘there is evidence that other patients could present with the same condition and equally benefit from the treatment, the request will be considered as a change in routine clinical commissioning policy instead’. Detailed information about IFRs is published by NHS England.