How the NHS commissions services for bleeding disorders
Haemophilia, von Willebrand’s disease and other bleeding disorders affect relatively few people but require a high level of clinical expertise and treatment with expensive drugs.
This makes it impractical for individual hospital trusts, health boards or clinical commissioning groups (CCGs) to provide a high standard of care for the low number of patients within their responsibility, or to manage the high cost of treatment. Health services therefore centralise their arrangements for commissioning to maximise clinical expertise in specialised treatment centres (or as part of a haematology department) and to optimise the efficiency of procuring drugs such as replacement clotting factors.
Commissioning is the process of assessing needs, planning services, procuring services and monitoring quality that ensures the cost effective delivery of care. Responsibility for commissioning specialist services in the home nations is devolved. In England, the responsible agency is NHS England. In Scotland, it is the National Services Division of NHS Scotland. In Wales, it is the Welsh Health Specialised Services Committee. In Northern Ireland, it is Health and Social Care.
Commissioning arrangements are most comprehensively provided by NHS England and therefore form the basis of this summary; national variations are detailed separately.
Commissioning in England
The King’s Fund has published a clear summary of the NHS commissioning process in England. This describes how most services are the responsibility of CCGs but specialised services, such as renal dialysis, blood disorders, neonatal services and treatments for rare cancers, are commissioned centrally. The commissioning structure is summarised in Figure 1.
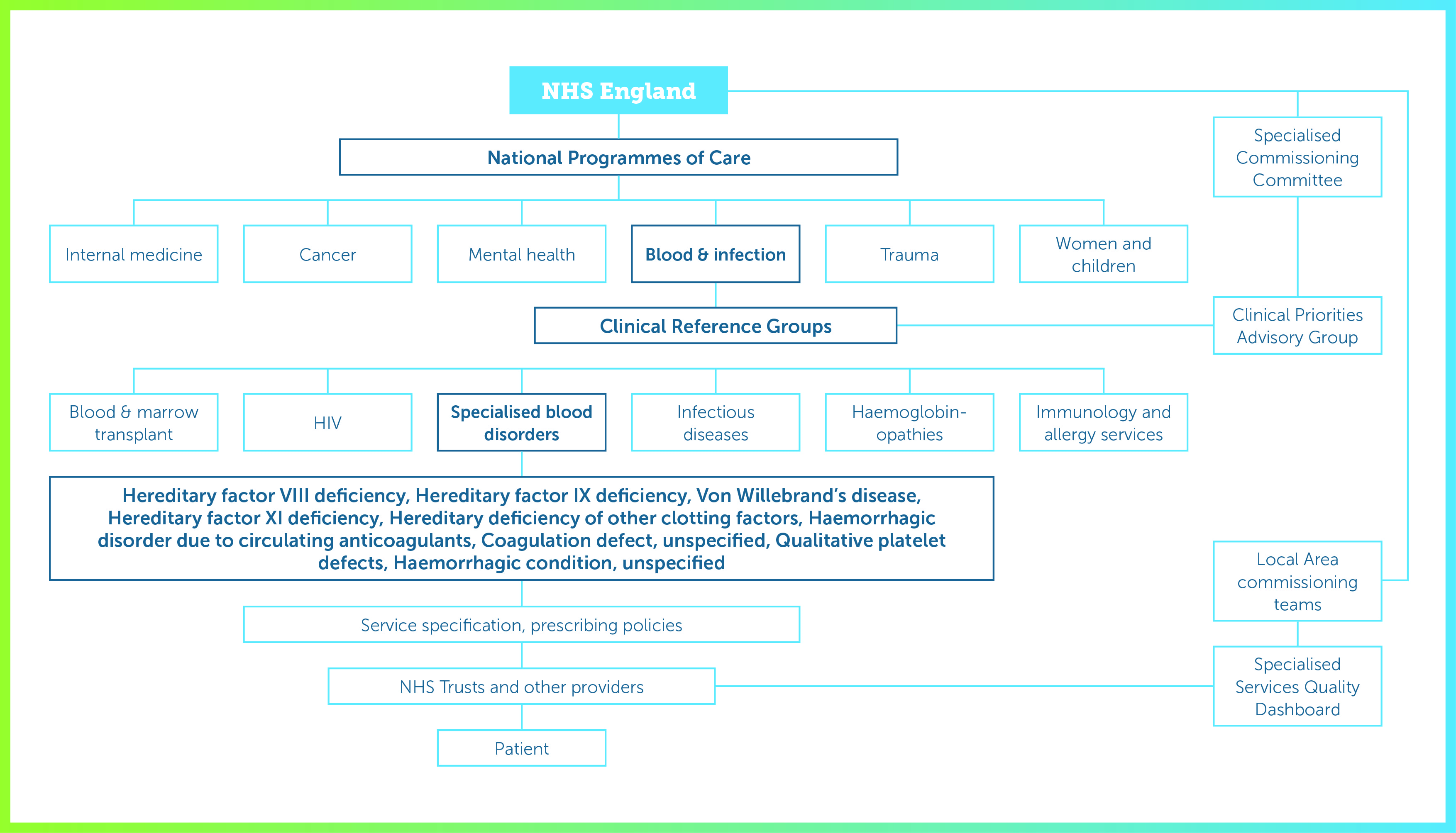
NHS England commissions specialised services through six National Programmes of Care, (NPoC) for internal medicine, cancer, mental health, trauma, women and children, and blood and infection. Each NPoC includes several Clinical Reference Groups (CRGs) to provide clinical advice and leadership on specific disorders. CRGs are encouraged to develop partnerships with the pharmaceutical industry.
NHS England describes CRGs as “groups of clinicians, commissioners, public health experts, patients and carers [who] use their specific knowledge and expertise to advise NHS England on the best ways that specialised services should be provided”.
CRGs lead on the development of clinical commissioning policies, service specifications and quality standards. They also provide advice on innovation, horizon scanning, service reviews and guide work to reduce variation and deliver increased value. CRGs, through their Patient and Public Voice (PPV) members, also help ensure that any changes to the commissioning of specialised services involve patients and the public.
The Blood and Infection NPoC includes CRGs for blood and marrow transplantation, specialised blood disorders, HIV, infectious diseases, haemoglobinopathies, and specialised immunology and allergy services. The CRG for specialised blood disorders covers haemophilia and other bleeding disorder services; within NHS England this means all care ‘provided by Specialist Haemophilia Centres including inpatient care where the cause of admission is related to a bleeding disorder. The service includes outreach when delivered as part of a provider network. This applies to provision in adults and children.’ The day-to-day process of commissioning is carried out by ten local area commissioning teams divided between the four NHS regions; this is intended to devolve decision-making as close to the clinical front line as possible.
Commissioning in Scotland
The population of Scotland is approximately 5 million (less than one-tenth that of England), and NHS Scotland takes a different approach to commissioning: specialist services are provided through its National Services Division (NSD). As in England, commissioning services for people with rare disorders aims to ensure equal access and optimise outcomes by securing funding, avoiding duplication and preventing excessive costs falling on an individual health board. Which services qualify for specialist status is determined by a national designation process.
Haemophilia services are commissioned at a national level from six haemophilia treatment centres (in Aberdeen, Dundee, Edinburgh, two in Glasgow and Inverness). On their behalf, NSD manages funding for clotting factor concentrates through a risk sharing agreement (details are not currently available online). The six centres work to a common protocol that specifies the provision of home delivery of factor concentrates, data management, stock management in hospitals and product administration procedures. These standards are set by the Scottish Inherited Disorders Bleeding Network (SIDBN), one of several national managed clinical networks in Scotland. SIDBN has responsibility for the care of people with haemophilia A, haemophilia B, von Willebrand disease, acquired haemophilia and other related bleeding disorders, and other rare forms of inherited bleeding disorders. It has three work streams: stakeholder engagement and communication; best practice, policies and protocols; and quality improvement, audit and data. However, online information is either out of date or not available.
The Scottish Medicines Consortium (SMC) is responsible for appraising new medicines for NHS Scotland. Factor replacement therapy normally falls outside the SMC’s remit, but it has the option of assessing a new technology if health boards need to know whether it is cost effective.
Commissioning in Wales
NHS Wales serves a population of approximately 3 million. Specialised services are overseen at a national level by the Welsh Health Specialised Services Care team (WHSSC) within a strategic framework that sets out the broad aims of ensuring equal access to cost-effective treatments. WHSSC, a joint committee of the Local Health Boards, has four directorates that cover patient care, medical, planning and finance, and corporate services via multidisciplinary Programme Commissioning Teams with six areas of responsibility (cancer and blood, cardiac, mental health, neurological and chronic conditions, renal, and women and children). The Cancer and Blood Programme includes inherited bleeding disorders.
WHSSC has a service specification for inherited bleeding disorders, which it defines as haemophilia A and B, von Willebrand disease and other rare inherited bleeding disorders, but including acquired haemophilia and other related bleeding disorders. The service specification develops the English model of a comprehensive care centre (one in Wales but Welsh patients are also served by two in England) and a haemophilia centre (two in Wales). The document describes the care pathway, standards for service quality and patient safety, and indicators for monitoring performance.
This is complemented by a policy on the management of patients with inherited bleeding disorders including haemophilia, which specifies the type of treatments (but not specific products) that will be funded (prophylactic blood products, on-demand therapy, home delivery, immune tolerance induction, and treatment for the complications of severe haemophilia), the criteria for treatment and how patients should be referred. Requests for individual funding for patients whose treatment is not covered by this policy are covered by a procedure similar to that for England.
WHSSC has also published a policy on the use of emicizumab within its licensed indication. This adopts the commissioning criteria developed by NHS England.
Commissioning in Northern Ireland
The population of Northern Ireland is approximately 1.8 million. Healthcare is the responsibility of Health and Social Care (HSC, the equivalent of the NHS), which in turn is accountable to the Department of Health, Social Services and Public Safety for Northern Ireland.
The HSC Board is responsible for commissioning services, managing resources and performance improvement for the five Health and Social Care Trusts (and also primary care and some community services). Planning and resourcing is carried out by the Board’s Local Commissioning Groups (LCGs), which cover the same geographical areas as the Trusts.
Specialist services are commissioned nationally in line with the principles of NHS England’s Commissioning for Quality and Innovation framework. Haemophilia care is delivered through a single provider (the Comprehensive Care Centre at Belfast City Hospital). Performance data are collected routinely. Specialist services in Northern Ireland are served by forums with representation from Trust management and clinicians, the HSC Board and the Public Health Agency. It is not clear from the information available whether haemophilia services are supported in this way.
Acknowledgements
The assistance of Anne Lee, Chief Pharmacist, Scottish Medicines Consortium, and Helen Manson, Haemophilia Centre and Thrombosis Unit, Belfast City Hospital, is gratefully acknowledged.