HNA 2017, Birmingham
Nurses make difficult decisions on a daily basis and many of these raise issues of consent, confidentiality and ethics. All of us, at one time or another, are uncertain which is the best course of action because it’s difficult to reconcile the conflicting needs of the people involved and our professional obligation to exercise a duty of care. In the first session of the 2017 HNA meeting, nurses presented four case studies that highlight how difficult ethical questions can arise in day-to-day practice.
Secrets, lies and deception – a case-based discussion about confidentiality, consent and ethics
To tell or not to tell?
April Jones, Nurse Specialist, Newcastle upon Tyne Hospitals NHS Foundation Trust, acknowledged that she was unsure how to resolve this case. She described a 47 year-old man, a religious leader in his local community, who has severe haemophilia A. He has eight children, of whom four are obligate carrier daughters ranging in age from 2 to 18. He previously had hepatitis but was now PCR-negative after antiviral therapy.
He is a fiercely private man and no-one knows about his haemophilia apart from his wife. He has good adherence with this thrice-weekly prophylaxis, which he self-administers after locking himself in a room.
In 2008, the haemophilia service wrote to his GP, stating: ‘“X’s children are unaware of his diagnosis of haemophilia or that he treats himself at home. X is aware that his daughters should have their factor VIII levels checked prior to any invasive procedures undertaken and that they will need to know about their obligate carrier status when they are older to allow informed decisions around their antenatal options”. This was repeated in 2015.
By 2017, he still had not informed his children and remains reluctant to do so. He may have unrealistic expectations of his eldest daughter’s behaviour and attitudes and it is possible that his daughters could enter an arranged marriage without knowing about their carrier status. It is also possible that their status might affect their perceived suitability in an arranged marriage.
Laura Boyes, Lead Consultant Genetic Counsellor in the West Midlands, suggested this case called for the use of counselling skills to explore:
- Why he is so fiercely private about his condition?
- Can we help gently normalise or challenge that?
- What is his wife’s perspective on disclosure?
Noting that often children are more aware than we give them credit for, she wondered whether they could have noticed or suspected something. She also proposed gently discussing the potential reasons to disclose:
- The daughters’ autonomy, their health risks if they have to undergo a procedure, and the reproductive risks
- What if… he were taken ill while only his daughters were there? How would they feel if they found out another way?
- The impact of secrecy and deception on the family
- The potential relief to everyone if they know about his haemophilia
She suggested he be asked to “Imagine how you might tell them”
- Would it help to talk about how you might go about it when the time is right?
- Who would be better telling your daughters– you or your wife? What might you say? Would it help to try it out here with me?
- Has you discussed this with your wife?
- How do you think the daughters would respond?
- Would any resources be useful?
- Would it be helpful if we met to discuss this again in X weeks?
I don’t quite know where I belong
Cathy Harrison, Haemophilia and Thrombosis CNS/ANP, Sheffield Teaching Hospitals NHS Foundation Trust, presented a case study that was almost a mirror image of the first: in this instance, a daughter was aware that her father had haemophilia but had not been told she’d been conceived by donor insemination.
The father had severe haemophilia A and was HIV- and HCV-positive. His daughter was born in 1993; in 2008, she was studying GCSE biology and becoming aware of the genetics of haemophilia. By then, her father had end-stage liver disease and was on the transplant list. The haemophilia service encouraged him to consider his daughter’s mental health wellbeing and tell her she was not at risk of being a carrier. The following year he did so; she accepted counselling from the centre to help her deal with this news. During counselling, she discussed the reasons for donor insemination and was offered the opportunity to meet other adolescents from families in similar circumstances. At this time she stated ‘I don’t know where I belong.’ There was little time left for reconciliation with her father, who died soon after.
Janet and John, an IVF Story
Being unaware of carrier status can have far-reaching implications. Simon Fletcher, Lead Research Nurse, Oxford University Hospitals NHS Foundation Trust, described the de novo presentation of a woman whose status came to light when her son presented with prolonged bleeding associated with a torn frenulum. She had conceived by IVF and in 2010 and 2013 had also donated eggs through an egg-sharing scheme to two fertility clinics. When the donations were tracked, it was found that one boy with haemophilia had been conceived at each clinic. Her remaining donated eggs were subsequently destroyed.
There are currently 1000 – 1500 new donor egg registrations per year and 500 – 1000 registrations in egg-sharing schemes. Donors undergo genetic screening for specific disorders but haemophilia is not among them. Should it be? And would screening be cost-effective?
Where does our duty of care stop?
Jayne Keaney, Haemophilia Nurse Specialist, Royal Liverpool and Broadgreen University Hospitals NHS Trust, raised the question of how a nurse should respond when faced with conflicting obligations. She described a 32 year-old HIV-positive man with mild haemophilia A who was being nursed in a side room. When she entered the room to talk to him, he was with a young woman who he asked to leave. The woman was visibly upset by this but the man insisted she was a friend, not his partner. Subsequently, the woman stated she had been his partner for the past 18 months; she said she had become unwell about four months previously and had not felt well since, raising the possibility she had seroconverted.
The issue came to the attention of the man’s father, who was well known to the haemophilia service. He believed that his son’s partner was aware of his HIV status but, once raised, the subject became a family matter and the partner was then informed. She was tested and found to be HIV positive. The couple eventually separated and both moved away.
Ten years later, the police informed the haemophilia service that the woman had brought a case of actual bodily harm against her former partner and Jayne was asked to give a witness statement. By this time, the man could not be interviewed because he was too ill; he died in 2016 and the case was dropped.
Genetic testing, consent and duty of care
Patients undergo tests and provide confidential information so that professionals can decide how best to treat and support them. To ensure patients fully disclose essential information, it is vital they feel reassured that what they reveal is kept confidential and secure. This is straightforward in the context of genetic testing until the results provide information that affects family members as well as the patient, said Laura Boyes, Lead Consultant Genetic Counsellor, West Midlands Regional Genetics Service.
In most instances, people are altruistic about sharing the results of genetic testing but the need to disclose information can raise questions such as Who’s information is it? What do we do if the patients is reluctant to share? Can this be shared without consent? and How do we support the patient?
The case of Veronica illustrates how conflicting needs create an ethical dilemma for health professionals.
Veronica has breast cancer; genetic testing identified a mutation that means her two sisters have a 50% chance of developing the condition. After her mum died, family relationships became strained with arguments over the house and money. She and her twin brothers felt forced to move out of the family home so that it could be sold. She felt that she was left with no support at a young age; she has not told her sisters of her diagnosis and was unwilling to share her genetic result with them.
The assumption that confidentiality is always paramount is as inappropriate as the assumption that disclosure is always permissible. There are four ethical principles at stake, as summarised in the figure:
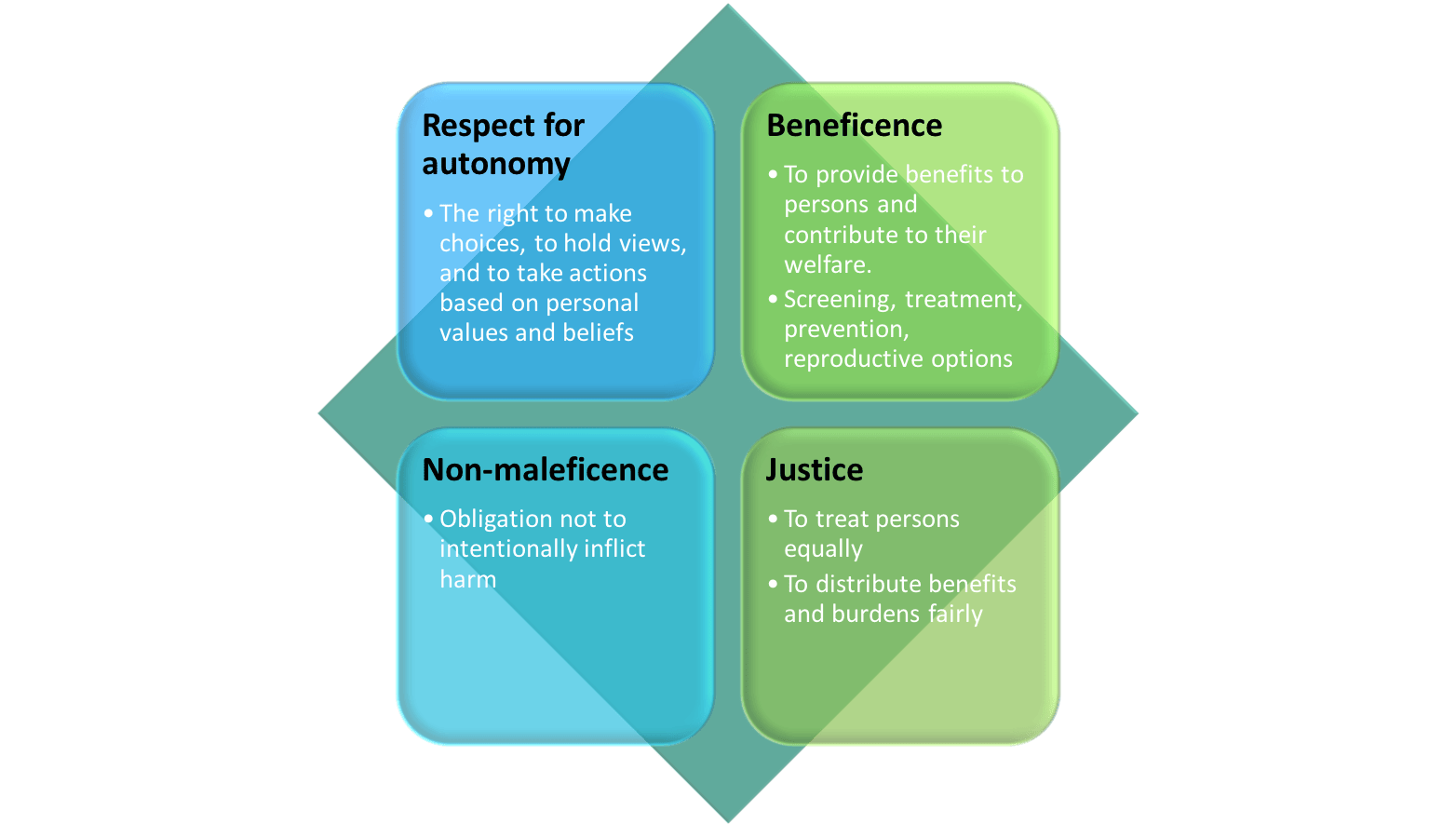
For Veronica, a balance must be struck between confidentiality and disclosure. The arguments for confidentiality include loyalty to the patient, her autonomy and right to privacy, the clinician’s obligation to avoid harm and the importance of preserving her trust. In favour of disclosure are recognition of family members’ and their right to know, the opportunity to benefit others, the need to treat people equally and the reality that delayed disclosure could lead to a breakdown in family relationships.
Guidance on decision-making
There is guidance on addressing the ethical issues raised by cases such as these. Lucassen and Parker proposed that because genetic information is familial, it is owned by a ‘joint account’ and the information should be shared with all account holders unless there are good reasons not to [1]. Article 8 of the European Convention on Human Rights recognises that the value of privacy needs to be balanced against the rights and freedom of others. It may be acceptable to interfere with such privacy provided that the interference is proportional to the protection afforded to others. The General Medical Council [2] and the British Society for Genetic Medicine [3] have published guidance for clinicians.
General Medical Council
‘Genetic and some other information about your patient might at the same time also be information about others the patient shares genetic or other links with.’
‘If a patient refuses consent to disclosure, you will need to balance your duty to make the care of your patient your first concern against your duty to help protect the other person from serious harm’.
British Society for Genetic Medicine
It is important to weigh up the harms of breaching confidentiality with the potential benefits to relatives and to the public interest of doing so. The rule of confidentiality is not absolute and, in special circumstances, it may be justified to break confidence where in doing so a serious harm can be avoided. Before doing so, a practitioner should generally:
- attempt to obtain consent for disclosure
- discuss the case with experienced professional colleagues
- inform the patient that they intend to breach this confidence and why
- limit any disclosure to that which is strictly necessary for the communication of risk
- document discussions with the reasons for disclosure
However, these issues may still come before the courts. In the case of ABC v. St George’s Healthcare NHS Trust and others [2015] EWHC 139, a man with Huntington’s disease did not allow doctors to inform his daughter of his diagnosis. She claimed that the failure to inform her was negligent and breached Article 8, and that it had caused psychiatric damage. If her daughter is also affected, she faces additional expense which (had she known) she would have avoided by having an abortion. The case was overruled but is currently under appeal.
Practical advice on promoting disclosure
At the pre-test counselling/consent stage it is useful to discuss the implications of the test for the wider family and the importance of passing on relevant information. If the patient is reluctant, explore their thinking – there are many reasons why people don’t agree to disclosure, from guilt and protectiveness to estrangement and denial. Build a trusting empathetic relationship with unconditional positive regard. The Joint Committee on Medical Genetics recommends that discussions during the consent process should cover:
- the potential benefit to family members
- the fact that communication of certain aspects of information to family members may therefore be recommended
- the means of contacting those at-risk family members [3]
Filling out the consent form can make this process easier because it conveys the impression that asking these questions is a routine part of the procedure. If a patient seems reluctant, the lack of an outright ‘no’ suggests the possibility of agreement later.
If the result has implications for family members, the patient should be encouraged to share the information. It’s useful to review the potential difficulties and for the patient to practise how the conversation might go. Relatives need written information and contact details for the counselling service and the patient should be offered follow up.
Talking to children
Parents feel guilty about passing on a problem and concerned they may be blamed. They are anxious to protect their children from worry or upset and they are uncertain about when to tell them and how much to say. They may worry that talking to their children will be difficult and distressing.
Informing children avoids them feeling confused and unsure how or what to ask. It helps to take naturally occurring opportunities for discussion, give information in small amounts, avoid lying and provide age-appropriate information. Channels of communication should be kept open. The evidence shows that children are more matter of fact about genetic risk than parents expect and, feeling valued and respected, they cope better if information is shared. In fact, children may worry more about parents than themselves [4,5].
Children will benefit from knowing. They can access support from relatives, friends and health professionals. They come to understand that parents being upset about gene alteration is not their fault and it helps the family talk about it – something that comes as a relief to parents and is ultimately easier than keeping secrets. Parents can be role models, giving their children insight into how to cope with the risk. Siblings have different needs, so it is important to determine what they understand at each stage.
Sources of information
The Society and Ethics Research Wellcome Genome Campus has a YouTube channel that offers a series of videos entitled Genetic Counselling in Action in which these issues are addressed through role playing.
The Genethics Club, a‘national forum for the discussion, by health professionals, of practical ethical problems encountered in the working lives of clinical genetics departments in the United Kingdom’ (https://www.ethox.ox.ac.uk/ethics-support/genethics-club) provides support for health professionals and ethical committees.
Summary
Talking to patients about genetic testing is usually straightforward but can sometimes be difficult. Nurses should promote openness and avoid secrecy. Unexpected disclosure is not likely to be easy and it comes best from a patient or family member. Patients should be supported to find the best time and the best way to tell their relatives. Nurses should utilise the multidisciplinary team and recognise that there are times when it is appropriate to break confidentiality. When that happens, tell the patient if you plan to disclose.
References
-
Parker M, Lucassen AM. Genetic information: a joint account? BMJ 2004;329:165-7.
-
General Medical Council. Good Medical Practice. 2013. (www.gmc-uk.org/guidance/good_medical_practice.asp)
-
British Society for Genetic Medicine. Consent and confidentiality in clinical genetic practice. Guidance on genetic testing and sharing genetic information. 2nd ed. September 2011. (www.bsgm.org.uk)
-
Metcalfe A, Plumridge G, Coad J et al. Parents’ and children’s communication about genetic risk: a qualitative study, learning from families’ experiences. Eur J Hum Genet 2011;19:640-6.
-
Rowland E, Metcalfe A. Communicating inherited genetic risk between parent and child: a meta-thematic synthesis. Int J Nurs Stud 2013;50:870-80.
Advances in genetic testing for inheritable bleeding disorders
Eighty per cent of mutations causing haemophilia occur during spermatogenesis but 40 – 50% of cases are inherited. Keith Gomez, Haemophilia and Thrombosis Centre, Royal Free Hospital, London, explained that genetic testing is now an integral part of haemophilia care, making it possible to predict the phenotype and the risk of developing inhibitors, confirm the severity of the bleeding disorder, identify carriers and offer pre-natal and pre-implantation genetic diagnosis (which is funded by the NHS to prevent severe cases). It is also increasingly valuable in developing new treatments such as modified concentrates and gene therapy.
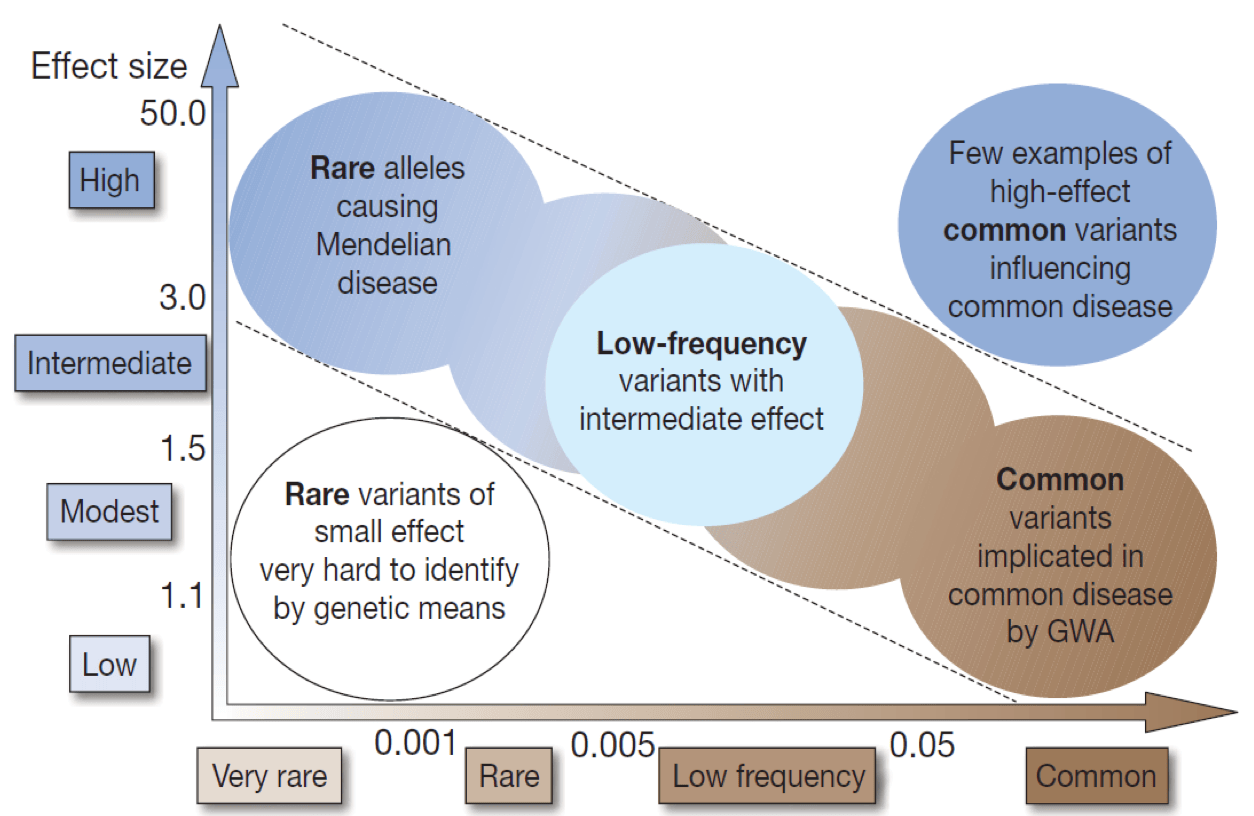
Variants: Not all genetic variants are important. Variants have differing impact on its function depending on the type of mutation. This is the major determinant of the risk of developing inhibitors: gross abnormalities are associated with highest risk (67% with deletion of multiple exons and 20% with intron 22 rearrangement) whereas missense mutations carry a risk of 5 – 10% [1]. How best to apply these findings in a clinical setting is an unresolved question: if we can identify a patient subgroup at increased risk of developing inhibitors, should they be treated differently – for example, for the first 50 exposures to factor replacement?
It is difficult to predict the impact of a variant – the normal human genome has 10 million of them – and testing by a gene panel and genome sequencing can identify many variants of unknown significance. Few common variants have a large effect but many common variants have a small effect; variants with large effect sizes are rare (Figure 1).
Testing for carrier status
Genetic testing is the only way to be certain about carrier status. Factor levels are unreliable in determining this: most carriers have normal levels even if they are carrying severe disease. By definition, there’s no doubt about which women are obligate carriers (daughters of haemophiliacs, mothers with an affected son and affected siblings or ancestors, and mothers with multiple affected sons are obligate carriers). But in other cases there is a strong possibility that a woman will not be affected – for example, the odds that a daughter of an obligate carrier is herself a carrier are 0.5 and for a mother of a sporadic case, the odds of being a carrier are 0.85.
The strategy for testing depends on the type of haemophilia. Haemophilia A is associated with inversions or large insertions or deletions in 43% of cases, with single nucleotide variations (SNVs) in 52%. All of these mutations may cause severe haemophilia whereas SNVs may also be associated with a mild disorder. The type of genetic test is therefore determined by haemophilia severity: mild and severe disorders require sequencing to detect SNVs whereas severe disorders also require polymerase chain reaction (PCR) techniques to detect inversions and multiplex ligation-dependent probe amplification (MLPA) for large insertions or deletions. Haemophilia B of all severities is associated with SNVs and large mutations, and testing requires the use of sequencing and MLPA.
Thrombogenomics (www.thrombogenomics.org.uk) is a gene panel for sequencing that is available free to people with haemophilia in the UK. It covers all genes associated with the coagulation cascade and platelet disorders and defines the severity of a bleeding disorder very clearly and accurately.
Recent developments in gene testing
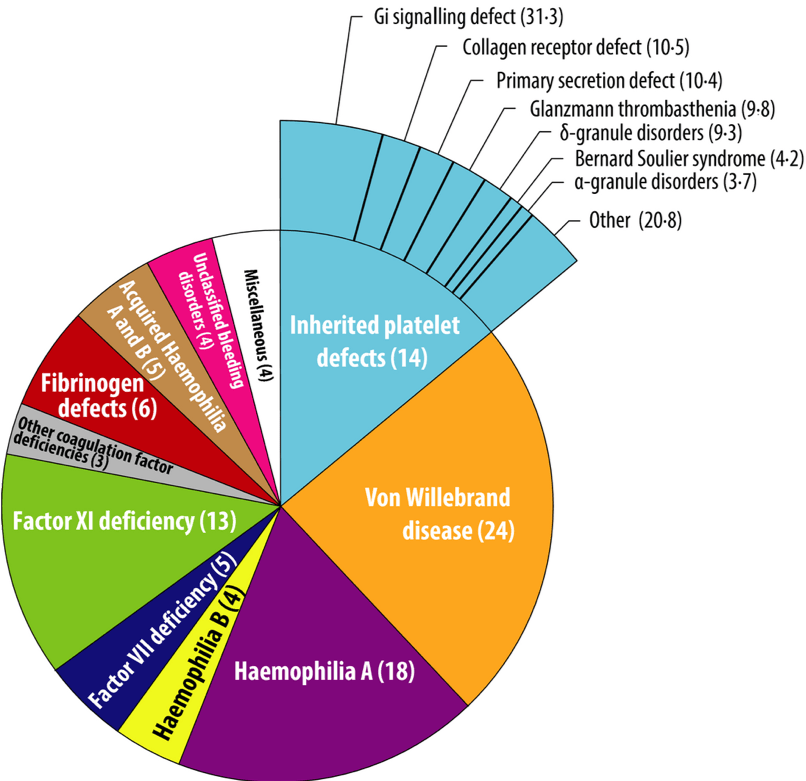
Gains in the management of haemophilia A have been rapid since Factor VIII was cloned in the 1980s but most patients attending a bleeding disorder clinic do not have haemophilia (Figure 2) and progress has been less encouraging in the management of other bleeding disorders. Rare bleeding disorders – of which about 7,000 have been described – pose a significant challenge. They have a strong genetic component and affect 1 per 1,000 of the population (more in areas of high consanguinity). The difficulties of managing these disorders are common to many other rare diseases, which collectively affect 1 in 17 people; of these, 75% are children and 30% die before their fifth birthday.
The Department of Health has developed a strategy for rare diseases that sets out its commitments to care and research, including the development of genetic testing [2]. It has established a universal database of genetic variants from healthy volunteers (e.g. blood donors), people with rare genetic disorders and patients with common disorders such as stroke; and a national biorepository of DNA, plasma samples and phenotype data. A biobank for bleeding disorders is now being piloted in seven vanguard centres in England; this will be evaluated shortly and rolled out nationally over the summer. In addition, Genomics England is developing a genomics service for the NHS: the 100,000 Genomes Project will sequence genomes from 70,000 people with a rare disease, plus their families, and patients with cancer with the aim of refining the diagnosis and developing new treatments (www.genomicsengland.co.uk).
Education
NHS staff need training to deliver genetic counselling. Health Education England is working to inform health professionals about the impact of genomics in healthcare. Its Genomics Education Programme (www.genomicseducation.hee.nhs.uk) includes a YouTube channel Genomics Education Programme (www.youtube.com/user/GeneticsEducation) with several videos explaining inheritance, genetic testing and the implications for personalised medicine.
Future challenges
Genetic testing should be available to everyone, which means it should be affordable, accessible to all clinicians and offered as part of the routine services from nurse specialists. Patients need to be informed if they are to participate: the Huntington’s Chorea Programme taught clinicians a salutary lesson when many people said they would take up the test but few actually did so. The challenge now facing geneticists is to assess the significance of the low impact variants that testing reveals and to identify disease modifiers at other loci.
References
-
Gouw SC, van den Berg HM, Oldenburg J et al. F8 gene mutation type and inhibitor development in patients with severe hemophilia A: systematic review and meta-analysis. Blood 2012;119:2922-34.
-
Department of Health. The UK Strategy for Rare Diseases. November 2013. (www.gov.uk/government/uploads/system/uploads/attachment_data/file/260562/UK_Strategy_for_Rare_Diseases.pdf)
Establishing the value of the Haemophilia Specialist Nurse
Patients rate specialists nurses highly, said Cathy Harrison (Sheffield Teaching Hospitals), and higher than any other health professional in terms of understanding patient needs, designing and implementing care pathways, obtaining patient feedback and being transparent and honest. Nurses are also cost effective: we deliver savings through reduced waiting times and duration of hospital stay; we help to avoid admissions and readmissions, freeing up consultant appointments; we are innovative; we provide education, specialist advice and services at the point of need; and we reduce treatment drop-out rates.
According to the Royal College of Nursing [1], commissioners, healthcare providers and the government need to recognise three facts if specialist nurses are to continue to deliver high quality, individualised care:
- every patient with a chronic or long term condition should have the right to specialist nursing care
- specialist nursing posts should be supported through robust long term funding
- specialist nurses need time to fulfil the key aspects of their role
Our medical colleagues, even those in our own departments, are often ignorant of our skills. This is partly because nurses have not been good at valuing what they do. It is not enough to describe our activities, we must put a value on them. In rheumatology, for example, routine follow up by specialist nurses has been shown to free consultants’ time to see new patients, saving £175,168 annually per nurse WTE [2]. A telephone helpline run by specialist rheumatology nurses avoided 60% of patients making an appointment with their GP [3]. Parkinson’s disease specialist nurses have been shown to deliver annual savings of £147,021 in bed days, £43,812 in avoided consultant appointments and £80,000 in unplanned admissions [4]. More recently, a London haemophilia service achieved a one-off saving of £200,000 when haemophilia specialist nurses managed a programme of dose reduction in prophylaxis in a small cohort of patients [5].
Specialist haemophilia nurses now face a new challenge: CQUIN is using Haemtrack to monitor haemophilia services even though this system does not accurately reflect our clinical work. It is time to quantify our activities and the forthcoming World Federation of Hemophilia 2018 Congress in Glasgow is an opportunity to showcase what we do. Activities can be coded using the freely available bioinformatics software package Pandora’s Toolbox (www.coding4medicine.com– registration is free). It would be useful for HNA members to quantify workload and the psychological impact of services on staff and patients for one month. Then we will be able to answer the question, ‘What difference did our intervention make to the patient?’
References
Royal College of Nursing. Specialist nurses. Changing lives, saving money. February 2010. (www.rcn.org.uk)
Royal College of Nursing. Clinical nurse specialists: adding value to care. April 2010. (www.rcn.org.uk)
Hughes RA, Carr ME, Huggett A et al. Review of the function of a telephone helpline in the treatment of outpatients with rheumatoid arthritis. Ann Rheum Dis 2002;61:341-5.
Parkinson’s UK. Parkinson’s nurses – affordable, local, accessible and expert care. 2011. (www.parkinsons.org.uk)
Greig A. How specialist nurse-led care can help to lower the costs of prophylaxis. J Haem Pract 2014;1:2-4. doi: 10.17225/jhp00002.
Bhave P, McGiffin D, Shaw J et al. Guide to performing cardiac surgery in patients with hereditary bleeding disorders. J Card Surg 2015;30:61-9.
Capturing cardiac care narratives
Life expectancy in people with haemophilia is increasing, said Jenna Stanley (Clinical Nurse Specialist, Haemophilia, St Thomas’ Hospital), and that means that haemophilia nurses are likely to encounter more age-related disorders such as cardiovascular disease. We have assumed that a bleeding disorder would confer protection against thromboembolic disease but there is little published experience of managing cardiovascular disorders. One exception is a case series of 17 patients undergoing cardiac surgery, in whom management focused on maintaining normal factor levels [6].
Ms Stanley described a survey of the management of 20 patients with bleeding disorders (severe in 4 cases) who underwent cardiac procedures including angiograms, angioplasty, pacemaker insertion and coronary stent placement. Thromboprophylaxis and antiplatelet regimes followed cardiac guidelines according to the procedure. Factor replacement strategies were varied, ranging from bolus dosing to continuous infusions; desmopressin use was not reported. Complications included one fatal bleeding episode, aneurysm (in the wrist), haemopneumothorax and bruising.
Two case studies, both in men with mild haemophilia A, illustrate contrasting demands on management. The first has been taking warfarin since undergoing aortic valve replacement in 1995; he received factor replacement between 2003 and 2006 but it was restarted in 2013 following admission for a serious thigh bleed. Anticoagulation is now being managed by the haemophilia team and he is currently being switched to a twice-weekly extended half life long acting FVIII. His heart valve will need replacing in a few years’ time.
The second was a 66 year-old man who underwent coronary artery bypass grafting in February 2016, supported by close liaison with between cardiologists, anaesthetists and the intensive care team. He received no thromboprophylaxis but took aspirin for >12 months. Perioperative factor replacement comprised continuous infusion (3iu/kg/hour reducing over 3 days) before switching to daily bolus dosing. He was discharged home with no bleeding problems but in March an inhibitor was identified, and he had bleeding complications and delayed healing of the graft site. He began treatment with immune tolerance induction and bypassing agents.
The nursing issues associated with cardiac procedures in patients with bleeding disorders included the additional amount of time required to manage each patient and the extra training required to use the continuous infusion pump. However, staff were supported by the multidisciplinary and cardiac teams and their confidence was high.
In conclusion, Ms Stanley said haemophilia nurses will see more patients with cardiovascular disease in the future and they will need the same interventions as people without bleeding disorders. It will be important to educate patients about cardiovascular risk and clarify the role of haemophilia nurses in delivering prevention strategies.
Managing patients closer to home
Sarah Johns (Haemostasis Clinical Nurse Specialist, Royal Cornwall Hospitals Trust) described the experience of the haemophilia treatment centre in Truro, which serves a resident population of 530,000 (increased by 55% in the summer) and an increasing student population, spread over a large geographical area that includes several island communities. The Trust routinely audits surgical outcomes, emergency attendances, patient satisfaction and waiting times. The service has to meet the usual standards for the Friends & Family Test and CQC inspections but there is no guidance on performance measurement or audit from the UK Haemophilia Centre Doctors’ Organisation. The up side of working in a small service that is remote from the nearest comprehensive care centre is that working relationships among the staff and with patients and their families are strong. Conversely, there is no capacity for cover when staff are away and the distance to specialist centres (in all specialties) makes access more difficult.
WORKSHOP 1: EHL factors – emerging themes from practice
This workshop, led by Simon Fletcher (Lead Research Nurse, Oxford University Hospitals NHS Foundation Trust) and Clare Ibbs (Clinical Nurse Specialist, Cardiff) and supported by Sobi, aimed to explore and share the practical experiences of patients and nurses in switching to extended half-life (EHL) products. The themes emerging from the discussion will be used to develop a checklist of key points to consider when switching to an EHL product, and to identify the resources needed to support the change.
Guidance on switching to EHL products has been published by NHS England and the UK Haemophilia Centres Doctors’ Organisation (UKHCDO). NHS England recommends that patients first have a pharmacokinetic assessment to determine the correct dose. The new treatment must be cost-equivalent (or cheaper) than what it replaces and result in at least one less infusion per week. Patients must have completed Haemtrack records for 6 – 12 months before switching and continue thereafter. If adherence is low or bleeding occurs, the patient should revert to their original treatment.
The UKHCDO recommends that patients should be made aware that switching to an EHL may not result in fewer doses. After switching, patients should be followed up every 4 weeks for 3 months to assess bleed patterns and trough levels. They should be screened for inhibitors after 10 EDs and at 3 months after switching or if clinically indicated, and assessed at 1 year for annualised bleeding rate, adherence, convenience, joint score and treatment cost.
How closely does clinical practice follow these guidelines? Anecdotal reports suggest experiences are mixed, with some patients welcoming a reduction in dose frequency and others wanting to remain with their familiar traditional product, whereas nurses say they have some patients with access problems who would benefit from fewer doses and others who are undergoing more tests associated with surgery because staff are not familiar with the new treatment.
Workshop participants were asked to consider and comment on three statements:
EHL products have been very popular with patients at our centre and we have managed to change a lot of the patients that we thought would benefit: Few agreed outright with this statement. Overall, participants felt they had too little experience so far – most had been waiting for publication of practice guidelines before starting to switch patients, so they were still in the early phases of the process.. Interpretation of the guidelines varies in different centres, with some implementing them strictly and others taking a wider perspective – for example, looking at overall rather than per patient reductions in costs. Those patients who have switched are, by and large, happy with the new products
I find it easy to explain the EHL products to patients and feel confident that they understand how they work: Most participants disagreed with this statement, though they did feel that patients who have been counselled understood what a longer half-life meant for them.
A patient on an EHL product rings your centre. They had treatment 2 days a go and they now have a bleed. You are confident with how this should be managed and can easily advise the patient: Only 4 of 30 participants stated they were confident managing a bleeding episode during treatment with an EHL product.
Participants then split into small groups to discuss the advantages and disadvantages of EHL products in three scenarios:
| Case study 1 | Case study 2 | Case study 3 |
| · Severe haemophilia B
· 77 years old · 1000IU recombinant FIX alternate days · No history of inhibitors |
· Severe haemophilia A
· 22 years old · 1000IU recombinant FVIII 3 times a week · No history of inhibitors |
· Severe haemophilia A
· 3 years old · 1000IU recombinant FVIII (Monday, Wednesday, Friday) · No history of inhibitors
|
| · Patient agrees to a PK on EHL product with a view to swap
· Five days after his first dose of product he attends for bloods and reports generally not feeling well · The doctor reviews him and is happy that it is not drug related |
· The patient is taking a sports degree at University and plays a lot of football
· He is keen to switch to an EHL product as he struggles with self treatment · He has a history of non-compliance with prophylaxis and often over treats when he has a bleed
|
· The patient’s parents do not self-treat so he doesn’t currently receive optimal prophylaxis (no weekend treatment)
· Parents do not complete Haemtrack · Parents demonstrate poor understanding of bleed recognition |
Their comments revealed a wide range of opinions reflecting both support for a new era of treatment and caution in appraising its benefits for patients. The issues identified included:
- Monitoring blood levels is time-consuming
- Medical knowledge/experience
- Switching requires more appointments and more patient education
- ‘Don’t fix it if it ain’t broke’
- Some patients may be reluctant to change
- Not all patients see an increase in half-life but for those who do EHLs mean:
- less venous access
- fewer injections
- improved quality of life and less time spent on treatment
- reduced risk of infection
- better cover, especially at weekends
- Many patients with haemophilia A are used to switching treatments; this is not usually the case for those with haemophilia B
- Haemophilia nurses do not always feel empowered to recommend prescribing EHL products to the MDT
In summary, the workshop revealed that many participants currently lack sufficient experience with EHL products to offer definitive advice. Given the caution about their use and uncertainty about their impact on management, it is perhaps useful to avoid calling EHL products as ‘better’ than older agents; instead, they should more accurately be described as ‘a different product that may benefit you’.
WORKSHOP 2: Multidisciplinary team working
Caring for a person with haemophilia is about more than managing a bleeding disorder. That individual lives with issues that affect physical health and functioning, psychological health and adjustment, social and environmental wellbeing and the expectations and beliefs of themselves and their families and carers. The multidisciplinary team (MDT) is seen as the best way to meet these needs and is the ideal management model in many specialties. In this workshop, organised by Grainne O’Brien, April Jones, Steve Classey and Nanda Uitslager and supported by Pfizer, HNA nurses contrasted their role as individual caregivers with that of being a member of the MDT in delivering patient-centred care.
The workshop considered three case studies to explore the interactions and boundaries between an individual clinician’s role and that of the MDT and specialist services. One group conceptualised patient-centred management on an astronomical scale, with various agencies in orbit around them. Closest was the family, next a haemophilia team comprising nurses, doctors, a physiotherapist, psychologist, play therapist, home delivery company, social worker, lab support and a clerical team. Specialist services such as orthopaedics, the pain team, pharmacy, and community, primary and secondary care occupy wider orbits. Each MDT member is orbited by their own agencies, many of which they share with other services and some, like the HNA, are specific to them.
What this model illustrates is that haemophilia nurses have very wide ranging interactions with health professionals across many sectors on a daily basis. The wider system is multifaceted and complex, requiring skill to negotiate, and all aspects are necessary if the patient is to receive the best holistic care. What that system delivers should be tailored to the needs and preferences of the person at its centre. Remember, said one workshop participant, this is a person, not a walking diagnosis – don’t forget that as you work hard doing your job. The patient’s desired outcomes might not match those of the MDT and it can be counter-productive to focus solely on haemophilia. It’s better to have a collaborative conversation with the patient at the start, addressing all the aspects of their lives that impact on, or are affected by, their care.
Others highlighted the importance of getting the whole of a patient’s story before starting to make decisions. Only then can they be referred to the MDT members best able to help. Their story will, of course, change over time. We all know that the MDT can be medically driven, so it should be recognised that every team member has an equal voice. For example, the MDT should not allow social care interventions to be overridden by a strong medical voice when both approaches are needed. Patients will be confused if they receive different or conflicting messages about what’s important and what they should do. A management strategy should be agreed at an early case review to ensure that MDT members are singing from the same hymn sheet.
Haemophilia services are usually blessed with effective MDTs but there is a risk that one team will try to manage every problem. It is important to look for support to the other services outside the MDT (those in wider orbits, to use the model proposed by the first group).
In summary, assessing a person’s current situation means showing empathy and using good communication skills to help us understand and to help them understand. We need to use our expertise to support, investigate distress, help with social situations, provide appropriate intervention, link in other supports and refer on if necessary. Everyone involved in managing long term conditions should be thinking holistically and we need to recognise our differences: some patients will require more support than others and staff will differ in the kind of support they feel able to offer.
We all hold different pieces of the puzzle and working together holistically provides the best patient-centred care. Each MDT member has a unique skill set and can support others in the team to see a case with a fresh pair of eyes. This helps us to deconstruct a situation and understand it differently so that we can manage complex management problems more effectively.
WORKSHOP 3: Finding Your Voice workshop
Public speaking can be daunting but it is a rewarding experience that is increasingly important for HNA nurses seeking to establish their professional role. This workshop, organised by Anne Wareing and Pamela Wick, and supported by CSL Behring, sought to provide some groundwork for nurses to help them communicate more effectively in multidisciplinary team meetings and, for those who want to speak at the WFH Congress next year, to present their research with confidence. This meant addressing themes such as the importance of being yourself, making yourself heard and the importance of body language.
Of course, not all public speaking is in a professional setting with colleagues or strangers – sometimes it’s a family celebration with people you know. Speeches have different functions and sometimes more than one: being persuasive (trying to get people to vote for you), informative (education, counselling), entertaining (a best man’s speech) or celebratory (introducing the winner of an award).
Whatever the purpose of their speech, people often rate public speaking among the worst fears they can imagine but there are tips that (with practice) make the experience less traumatic.
- Prepare for your speech – The more you practice and prepare the more comfortable you will be
- Visualise your success – If you close your eyes and visualise yourself successfully delivering the speech, you will trick your brain into believing you’ve done it before and you won’t feel as nervous
- No-one can see you are nervous – most of your nervousness is invisible to the audience, who believe you are just calmly presenting your speech
- Mistakes are going to happen – Your audience will only realise you’ve made a mistake if you draw to their attention to it; take a deep breath, collect your thoughts and carry on as if nothing happened
It is important to be yourself when you speak because your audience will pick up your verbal and non-verbal signals and they need to be assured that you are speaking with an authentic voice. If you are comfortable, speak clearly and consistently with transparency and openness, your audience will think you are knowledgeable and have integrity and vision. They will find you engaging, motivational and knowledgeable. These skills are equally applicable to small and large meetings.
Of course, the point of public speaking is to be heard and to influence your audience. You can make your speech more effective by using several techniques:
- Show that you believe what you’re saying, then people will believe you
- Pay attention to the verbal and non-verbal signals (including ‘power poses’) of others so you know how they’re thinking; be aware of their feelings and emotions; be compassionate
- Believe that you will succeed
- Keep going
- Build rapport with your audience by finding common ground, developing bonds and being empathic
- Engage your audience by aligning your interactions with their needs and feelings
Good public speakers come in diverse shapes and sizes. They include people like Steve Jobs, the late head of Apple; Nicola Sturgeon, Scotland’s First Minister; and Colin Moon, the star turn at last year’s HNA Annual meeting in Manchester whose style was the epitome of relaxed presentation. What they have in common is experience and practice.
Participants divided into groups to discuss their hopes and fears then, with the necessity of some public speaking, shared their conclusions. They agreed:
- The first presentation is always the hardest
- Delivering a presentation with a colleague can help to calm nerves
- Where you stand during a presentation can make a difference. For example, standing on a large stage can be intimidating
- It’s about confidence, knowing your subject and audience
- Once you have started your presentation, you can often relax into it
- In MDT meetings, it can be hard to make your point in the right way
- You learn from experience to prepare for MDT meetings
- It can be frustrating when you think of something you wanted to say after an MDT meeting
- Presenting at the HNA meeting can be a good stepping stone to prepare you for larger congresses because it is informal and presenters are talking to their peers. The snippety bits section is particularly good as it only requires a 5-minute presentation
- Some people see this differently – they feel more nervous presenting at an HNA meeting because they’re talking to peers with a lot of experience and they’re worried about being shown up
Having recognised the challenges, what issues should a speaker consider to be sufficiently engaged to speak in team meetings and to make the experience more comfortable?
- Ensure a meeting has a structure or agenda and that everyone has a chance to speak
- Familiarity and understanding of each other’s personalities
- Relationships and past experiences with other team members
- The mood of the participants
- Need to know when not to discuss things
- Prepare and know the subject matter
- Participants should have respect for one another’s thoughts and opinions so that everyone can speak without the fear of being judged
- On a personal level, a speaker should have self confidence, be positive and have good listening skills.
With the WFH Congress in Glasgow in the back of everyone’s mind, workshop participants discussed what a one-day training session on public speaking might include. The list was short but captured the key challenges facing everyone who takes the podium:
- What to do and what not to do
- Getting balance between what’s written on the slide and what you say
- How to deal with curve ball questions
- What to do if your mind goes blank
Until that HNA training session, here are some suggestions for further reading and viewing:
